MATERIALS
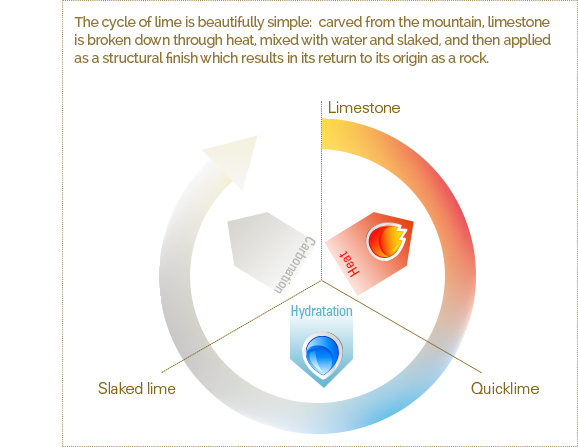 After processing, products derived from limestone have the unique ability to return to their original chemical form. Lime starts from rock form and goes back again to the state of stone, after a series of events that transform it, eventually recreating its natural mineral essence, without affecting its durability and permeability. Lime mortars are derived from limestone. When limestone is heated, it breaks down to form quicklime and releases carbon dioxide. Quicklime reacts with water to form calcium hydroxide, a process also known as slaking or hydrating. Slake lime is mixed into a thick slurry with sand and water to form various kinds of mortars for building purposes. When masonry has been laid the slaked lime in the mortar slowly begins to react with carbon dioxide to form limestone. The reabsorption of carbon dioxide from the atmosphere by the calcium hydroxide is time consuming and can take many years to form calcium carbonate (limestone). This process is known as ‘The Lime Cycle.’
After processing, products derived from limestone have the unique ability to return to their original chemical form. Lime starts from rock form and goes back again to the state of stone, after a series of events that transform it, eventually recreating its natural mineral essence, without affecting its durability and permeability. Lime mortars are derived from limestone. When limestone is heated, it breaks down to form quicklime and releases carbon dioxide. Quicklime reacts with water to form calcium hydroxide, a process also known as slaking or hydrating. Slake lime is mixed into a thick slurry with sand and water to form various kinds of mortars for building purposes. When masonry has been laid the slaked lime in the mortar slowly begins to react with carbon dioxide to form limestone. The reabsorption of carbon dioxide from the atmosphere by the calcium hydroxide is time consuming and can take many years to form calcium carbonate (limestone). This process is known as ‘The Lime Cycle.’
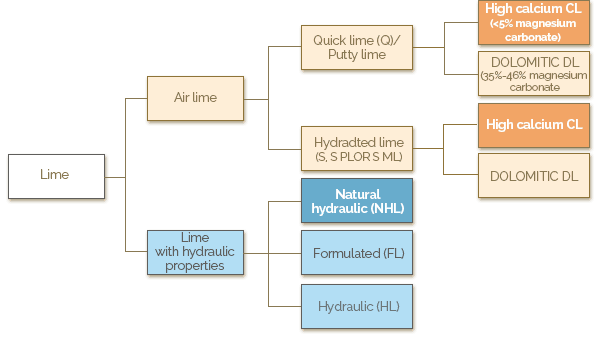 Quicklime is a white to gray solid with a crystalline structure, and the product of calcination of limestone. It is highly reactive with water, generating considerable heat in the hydration process. The primary forms of Quicklime are:
Quicklime is a white to gray solid with a crystalline structure, and the product of calcination of limestone. It is highly reactive with water, generating considerable heat in the hydration process. The primary forms of Quicklime are:
- high calcium quicklime derived from limestone containing up to 5% magnesium carbonate
- dolomitic quicklime derived from limestone containing up to 35% of magnesium carbonate
- hydraulic lime is derived from clay rich in silica, aluminum and iron and gets harder with the addition of water. It has more impurities and is less white.
 |
Environmental / Ecological
|
 |
Durability / Efficiency
|
 |
Health
|
 |
Aesthetics
|




